Investigation reveals FDA raised flags about continued Paragard IUD breaks in 2022
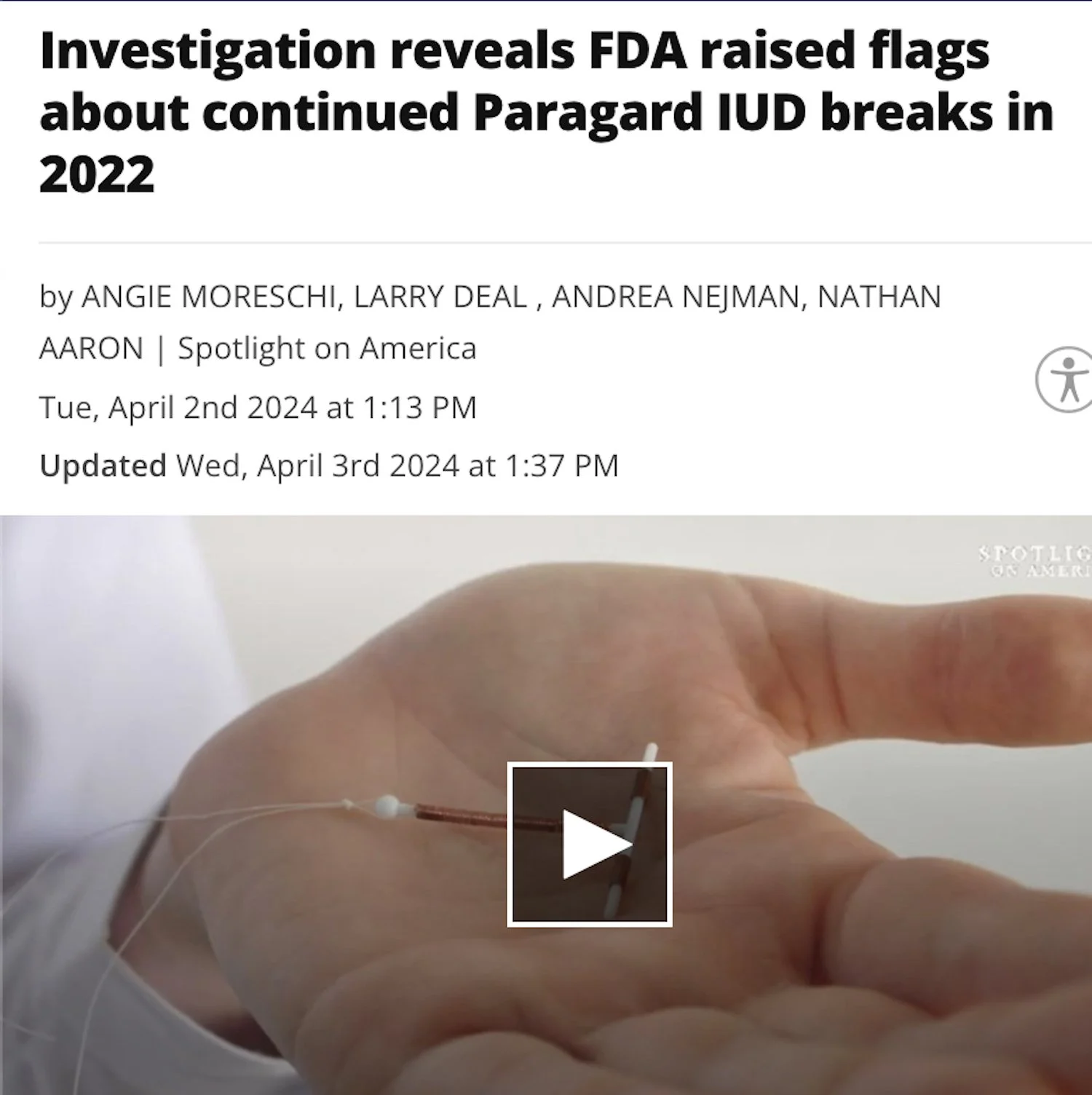
/ Internet Link“For more than three years, Spotlight on America has been raising concerns about the IUD Paragard as thousands of women have reported breakages with the device.
Since last reporting on this issue in September 2023, the Spotlight team discovered that the Food and Drug Administration (FDA) conducted an inspection of the Buffalo, N.Y. plant where the IUD is made by CooperSurgical.
The inspection spawned a highly critical report by the Department of Health and Human Services of the manufacturer, but there has been no movement by the company to address the breakages and the subsequent rising number of complaints.”
“BREAKAGE REPORTS SURGE
According to a Spotlight on America analysis of the FDA's Adverse Event Reporting System there were 1,231 reports of breakage in 2023. That's the most recorded in a single year since tracking began.
There have been more than 6,000 total complaints since tracking began, and 80% of them were deemed to be 'serious.'
As Spotlight on America noted last year, a 2022 analysis of all IUDs showed those made by Paragard break at a rate of nearly double compared to all other.
While Paragard's current warning label, issued in 2019, does acknowledge the possibility of breakage -- disclaiming "Paragard can break" and "Breakage ... can make removal difficult" -- the women the Spotlight team interviewed said they were never warned by their doctors about the potential when they were making a decision about birth control.“

















RSD Law
May 22, 2024